Latest Podcasts
Latest Updates From OncoNexus
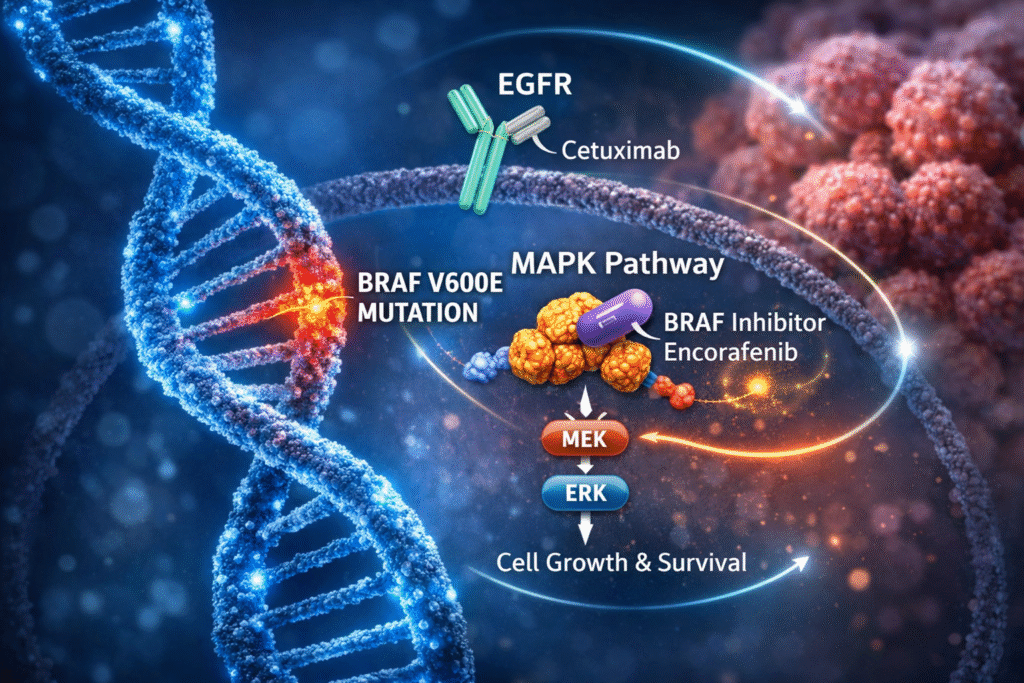
BREAKWATER Trial: Encorafenib Plus Cetuximab With Chemotherapy Receives FDA Traditional Approval for BRAF V600E–Mutated Metastatic Colorectal Cancer
The phase III BREAKWATER trial evaluated early integration of targeted therapy with encorafenib plus cetuximab in combination with chemotherapy for...
Head-to-Head PSMA PET Comparison Shows Lower Urinary Activity and Higher Detection Rates With 18F-flotufolastat vs 18F-piflufolastat in Low-PSA Recurrence
Prostate-specific membrane antigen (PSMA) PET imaging plays a central role in evaluating biochemical recurrence after radical prostatectomy. Yet, high urinary...
Emerging Options for High-Risk Non–Muscle Invasive Bladder Cancer
Dr. Katie Murray highlights the expanding treatment landscape for high-risk non–muscle invasive bladder cancer, especially for patients who are unresponsive...
ASCO GU 2026 Preview: HIF Inhibitors Redefine Kidney Cancer
Dr. Brian Rini previews major kidney cancer data at ASCO GU, led by positive phase 3 LIGHTSPARK trials highlighting the...
AI, Immunotherapy, and Early Intervention: Charting Oncology’s 2026 Trajectory
As oncology enters 2026, the field is shifting from reactive treatment toward anticipatory, precision-driven care. In this article, experts highlight...
PANOVA-3 FDA Approval: Tumor Treating Fields Plus Gemcitabine/Nab-Paclitaxel Improves Overall Survival in Advanced Pancreatic Adenocarcinoma
The United States Food and Drug Administration (FDA) has approved Tumor Treating Fields (TTFields) therapy delivered via the Optune Lua...
Latest Updates in Genitourinary Oncology
Head-to-Head PSMA PET Comparison Shows Lower Urinary Activity and Higher Detection Rates With 18F-flotufolastat vs 18F-piflufolastat in Low-PSA Recurrence
Prostate-specific membrane antigen (PSMA) PET imaging plays a central role in evaluating biochemical recurrence after radical prostatectomy. Yet, high urinary...
Emerging Options for High-Risk Non–Muscle Invasive Bladder Cancer
Dr. Katie Murray highlights the expanding treatment landscape for high-risk non–muscle invasive bladder cancer, especially for patients who are unresponsive...
ASCO GU 2026 Preview: HIF Inhibitors Redefine Kidney Cancer
Dr. Brian Rini previews major kidney cancer data at ASCO GU, led by positive phase 3 LIGHTSPARK trials highlighting the...
POTOMAC Trial: Durvalumab Plus BCG Improves Disease-Free Survival in BCG-Naïve High-Risk NMIBC
High-risk non-muscle-invasive bladder cancer (NMIBC) remains a clinically challenging disease state despite intravesical bacillus Calmette-Guérin (BCG), with substantial rates of...
LITESPARK-022: Adjuvant Pembrolizumab Plus Belzutifan Improves Disease-Free Survival in Resected Clear Cell RCC
LITESPARK-022 is a global phase III study evaluating the addition of belzutifan to pembrolizumab in the adjuvant treatment of patients...
Cxbladder Triage in the Evaluation of Microhematuria
Microhematuria is a common clinical finding that frequently prompts urologic evaluation, yet the incidence of underlying urothelial malignancy remains low,...
Latest Updates in Breast Cancer
2026 Advances and Ongoing Challenges in HR-positive, HER2-Negative Metastatic Breast Cancer
Recent advances have reshaped the management of HR-positive, HER2-negative metastatic breast cancer, with CDK4/6 inhibitors, genomically driven targeted therapies, and...
Maintenance Therapy Intensification with Palbociclib Improves PFS in HR-Positive, HER2-Positive Metastatic Breast Cancer
In the phase 3 PATINA trial, adding the CDK4/6 inhibitor palbociclib to maintenance HER2-targeted and endocrine therapy significantly prolonged progression-free...
FDA Approves Trastuzumab Deruxtecan (T-DXd) Plus Pertuzumab for First-Line HER2-Positive Metastatic Breast Cancer
The FDA has approved fam-trastuzumab deruxtecan-nxki (T-DXd; Enhertu) plus pertuzumab for first-line treatment of unresectable or metastatic HER2-positive breast cancer....
Can Multimodal AI Be Trusted in the Clinic? New SABCS 2025 Data Support Its Role in Personalized Breast Cancer Care
At SABCS 2025, new data evaluated whether multimodal artificial intelligence (MMAI) can be trusted to support clinical decision-making in early-stage...
SABCS 2025: Redefining Endocrine-Based Care in HR+, HER2-Negative Breast Cancer Across Early and Metastatic Settings
Key data from SABCS 2025 highlight how endocrine-based, biology-driven strategies continue to reshape the management of ER-positive, HER2-negative breast cancer....
SABCS 2025: Transforming HER2+ Breast Cancer Care: Key Updates from HER2CLIMB-05, DESTINY, and PHILA Trials
This article reviews recent pivotal HER2+ breast cancer trials, highlighting oral TKIs, antibody-drug conjugates, and chemotherapy-sparing strategies. Key studies—including HER2CLIMB-05,...
Latest Updates in Lung Cancer
Advances in EGFR-Mutated NSCLC: Balancing Efficacy and Safety in Combination Therapies
Recent studies, including FLAURA2 and MARIPOSA, show that combination therapies are improving survival for patients with EGFR-mutated NSCLC, though higher...
Osimertinib Plus Platinum–Pemetrexed Improves Overall Survival in EGFR-Mutated Advanced NSCLC (FLAURA2)
Final results from the phase III FLAURA2 trial demonstrate that adding platinum–pemetrexed chemotherapy to first-line osimertinib significantly improves overall survival...
FDA Grants Approval for Subcutaneous Amibantamab (Rybrevant Faspro) in EGFR-Mutated NSCLC
The FDA has approved subcutaneous amivantamab (Rybrevant Faspro) for adult patients with advanced or metastatic EGFR-mutated NSCLC. In the Phase...
FDA Grants Traditional Approval to Tarlatamab-dlle for ES-SCLC After Phase 3 Survival Benefit
The FDA has granted full approval to Tarlatamab-dlle (Imdelltra) for patients with extensive-stage small-cell lung cancer progressing after platinum therapy....
AI Model Predicts EGFR Mutations from Pathology Slides in Real-Time Silent Trial for Lung Adenocarcinoma
AI can extract critical genetic insights directly from routine pathology slides, streamlining decisions and accelerating targeted therapy access.
FDA Approves First Maintenance Therapy for ES-SCLC: Lurbinectedin Plus Atezolizumab Extends Survival in IMforte Trial
FDA approves lurbinectedin plus atezolizumab for ES-SCLC maintenance, extending OS to 13.2 months vs 10.6 months with atezolizumab alone (HR...
Latest Updates in GI Cancer
BREAKWATER Trial: Encorafenib Plus Cetuximab With Chemotherapy Receives FDA Traditional Approval for BRAF V600E–Mutated Metastatic Colorectal Cancer
The phase III BREAKWATER trial evaluated early integration of targeted therapy with encorafenib plus cetuximab in combination with chemotherapy for...
PANOVA-3 FDA Approval: Tumor Treating Fields Plus Gemcitabine/Nab-Paclitaxel Improves Overall Survival in Advanced Pancreatic Adenocarcinoma
The United States Food and Drug Administration (FDA) has approved Tumor Treating Fields (TTFields) therapy delivered via the Optune Lua...
HERIZON-GEA-01: Zanidatamab Plus Chemotherapy ± Tislelizumab Advances First-Line Treatment in HER2-Positive Gastroesophageal Adenocarcinoma
HERIZON-GEA-01 is a pivotal phase III trial evaluating zanidatamab-based regimens, with or without the PD-1 inhibitor tislelizumab, compared with trastuzumab...
Phase III COMMIT Study Shows Promising Frontline Approach for dMMR/MSI-H Metastatic Colorectal Cancer
The phase III COMMIT trial demonstrates that intensifying first-line immunotherapy with chemotherapy and antiangiogenic therapy can substantially improve outcomes for...
BREAKWATER Cohort 3: Early Integration of Encorafenib–Cetuximab Plus FOLFIRI Improves Response in BRAF V600E mCRC
Patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC) represent a biologically distinct subgroup with historically poor outcomes and limited first-line,...
Perioperative Durvalumab Plus FLOT Improves Survival in Resectable Gastric and GEJ Cancer
The FDA has approved AstraZeneca’s Imfinzi (durvalumab) in combination with FLOT chemotherapy as the first and only perioperative immunotherapy for...
Latest Updates in Oncology
AI, Immunotherapy, and Early Intervention: Charting Oncology’s 2026 Trajectory
As oncology enters 2026, the field is shifting from reactive treatment toward anticipatory, precision-driven care. In this article, experts highlight...
ASCENT-03: Trodelvy Plus Pembrolizumab Demonstrates Significant PFS Benefit in First-Line Metastatic Triple-Negative Breast Cancer
ASCENT-03 trial shows Trodelvy plus pembrolizumab significantly improved PFS vs chemo plus pembrolizumab in first-line mTNBC patients.
FDA Approves First Maintenance Therapy for ES-SCLC: Lurbinectedin Plus Atezolizumab Extends Survival in IMforte Trial
FDA approves lurbinectedin plus atezolizumab for ES-SCLC maintenance, extending OS to 13.2 months vs 10.6 months with atezolizumab alone (HR...
FDA Grants Priority Review to Enhertu Plus Pertuzumab for First-Line HER2+ Metastatic Breast Cancer
FDA grants Priority Review for Enhertu plus pertuzumab in first-line HER2+ metastatic breast cancer, with decision expected April 2025.
FDA Approves Gemcitabine Intravesical System (Inlexzo) for BCG-Unresponsive Bladder Cancer
FDA approves gemcitabine intravesical system for BCG-unresponsive bladder cancer, showing 82% complete response rate in SunRISe-1 trial.
FDA Approves Modeyso as First Treatment for H3 K27M-Mutant Diffuse Midline Glioma
Modeyso, the first treatment option for this ultra-rare and aggressive brain tumor, is commercially available in the US.