
Anixa’s FSHR-targeting CAR-T therapy demonstrates favorable safety profile at 3 million CAR-positive cells/kg (30-fold dose increase) in recurrent ovarian cancer patients, with no dose-limiting toxicities observed in the fourth cohort to date. The novel CER-T technology uses FSH as natural ligand rather than antibody fragments, representing a differentiated approach to CAR-T therapy.

Study Design & Population
- Study Type: First-in-human Phase 1 dose escalation trial (NCT05316129)
- Population: Adult women with recurrent ovarian cancer after ≥2 prior therapies
- Current Status: Second patient treated in fourth dose cohort
- Setting: Moffitt Cancer Center collaboration
- Primary Objectives: Safety evaluation, maximum tolerated dose determination, initial efficacy signals
Key Findings
- Dose Escalation: Fourth cohort receiving 3 million CAR-positive cells/kg (30x initial dose)
- Safety Profile: No dose-limiting toxicities observed in fourth cohort to date
- Technology: FSHR-targeting CAR-T using natural FSH ligand (CER-T technology)
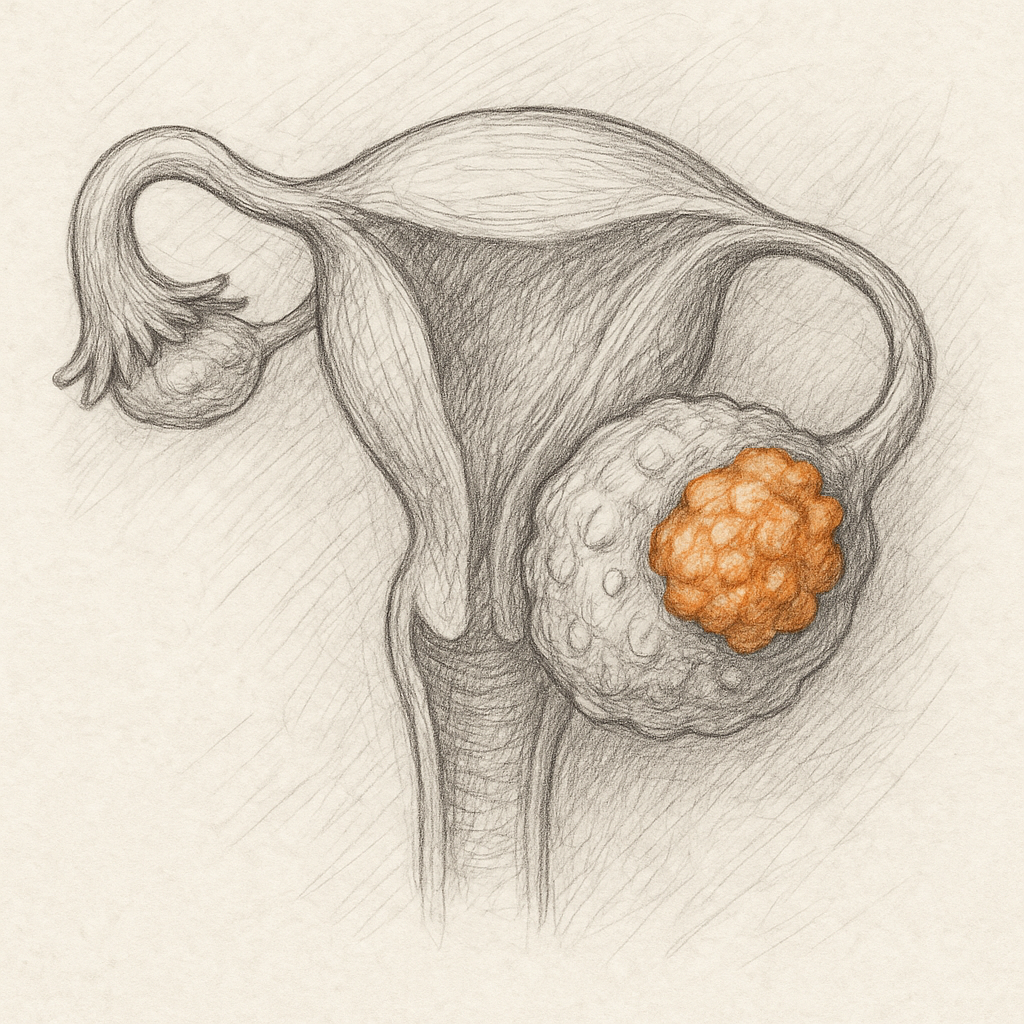
- Target Expression: FSHR selectively expressed on ovarian cells, tumor vasculature, and cancer cells but not healthy tissue
Clinical Implications
- Continued dose escalation suggests potential for therapeutic efficacy at higher cell doses
- Novel CER-T approach may offer differentiated safety/efficacy profile compared to traditional CAR-T
- FSHR targeting represents innovative approach for ovarian cancer immunotherapy
- Technology platform may have broader applications beyond ovarian cancer
Limitations
- Early-stage Phase 1 data with limited patient numbers
- Primary focus on safety rather than efficacy assessment
- No comparative data versus standard CAR-T approaches
- Preliminary results require validation in larger cohorts