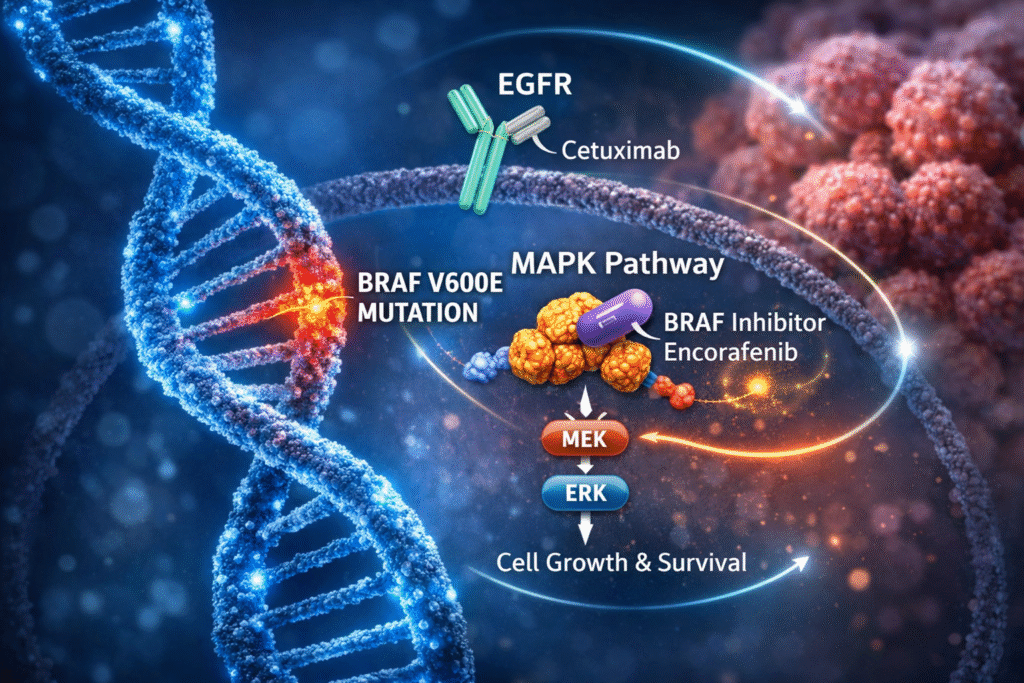

Patients with BRAF V600E-mutant metastatic colorectal cancer (mCRC) represent a biologically distinct subgroup with historically poor outcomes and limited first-line, biomarker-driven treatment options. Within this context, the Phase III BREAKWATER trial is evaluating early integration of targeted BRAF inhibition (encorafenib) plus EGFR blockade (cetuximab) alongside standard chemotherapy backbones in this high-risk population.

Cohort 3 evaluated the efficacy and safety of encorafenib plus cetuximab in combination with fluorouracil, leucovorin and irinotecan (FOLFIRI) versus FOLFIRI with or without bevacizumab in patients with previously untreated BRAF V600E-mutant mCRC.
Clinical Takeaway
In treatment-naïve BRAF V600E-mutant mCRC, adding encorafenib and cetuximab to FOLFIRI significantly improved objective response rates versus standard FOLFIRI-based therapy, without a meaningful increase in toxicity. These findings support flexibility in chemotherapy backbone selection while reinforcing the importance of early targeted therapy in this population.
Drug Profile & Mechanism
- Agent:
- FOLFIRI
- encorafenib
- cetuximab
- bevacizumab (control arm option)
- Class & Mechanism of Action:
- FOLFIRI: Combination cytotoxic chemotherapy consisting of:
- fluorouracil (antimetabolite; thymidylate synthase inhibitor)
- leucovorin (chemotherapy modulator enhancing fluorouracil activity)
- irinotecan (topoisomerase I inhibitor causing DNA damage)
- encorafenib: Small-molecule BRAF kinase inhibitor selectively targeting BRAF V600E, suppressing aberrant MAPK pathway signaling
- cetuximab: Chimeric monoclonal antibody targeting epidermal growth factor receptor (EGFR) inhibiting downstream proliferative and survival signaling
- bevacizumab: Monoclonal antibody targeting vascular endothelial growth factor (VEGF), inhibiting tumor angiogenesis
- FOLFIRI: Combination cytotoxic chemotherapy consisting of:
- Route of Administration & Dosing Schedule:
- FOLFIRI: intravenous (IV); every 2 weeks per standard protocol (fluorouracil bolus and continuous infusion with leucovorin and irinotecan)
- encorafenib: 300 mg orally once daily
- cetuximab: IV infusion administered weekly or every 2 weeks per standard dosing protocol
- bevacizumab: IV infusion every 2 weeks (control arm)
Target Population
- Adults with previously untreated metastatic colorectal cancer harboring a BRAF V600E mutation, as detected by an FDA-approved test
Phase III BREAKWATER Trial Study Design – Cohort 3 Specific (NCT04607421)
- Study Type: Randomized, international, open-label, phase III clinical trial
- Population: Cohort 3 enrolled 147 previously untreated patients with BRAF V600E–mutant mCRC
- Randomization:
- Experimental arm:
- encorafenib + cetuximab + FOLFIRI (n=73)
- Control arm:
- FOLFIRI +/- bevacizumab (n=74)
- Experimental arm:
- Follow-Up Duration: Ongoing
- Median follow-up: approximately 10 months at time of analysis
- Estimated study completion: 2027
Endpoints
- Primary Endpoint:
- Objective Response Rate (ORR) assessed by blinded independent central review (BICR)
- Secondary Endpoints:
- Duration of response (DoR)
- Progression-free survival (PFS)
- Overall survival (OS)
- Safety and tolerability
- Endpoint Maturity:
- ORR and early DoR data are available for Cohort 3
- PFS and OS analyses remain immature, with follow-up ongoing
Efficacy Outcomes
- Primary Endpoint Results:
- ORR
- Encorafenib + cetuximab + FOLFIRI: 64.4%
- FOLFIRI +/- bevacizumab: 39.2%
- ORR
- Time to response: Responses were observed early in both treatment arms, with tumor responses occurring at a median of approximately 7 weeks
- DoR
- Median DoR
- Combination therapy: Not estimable (NE)
- Control arm: 7 months (upper bound not estimable)
- Responses lasting ≥6 months:
- Combination therapy: 57.4%
- Control arm: 34.5%
- PFS:
- Data immature; analysis ongoing
- OS:
- Data immature, descriptive analysis ongoing
- Median DoR
Regulatory Milestones
- Approval Status: Not FDA approved for encorafenib + cetuximab + FOLFIRI
- Current Standard of Care:
- FOLFIRI +/- bevacizumab
- Encorafenib + cetuximab + mFOLFOX6 (accelerated approval, December 2024)
- Regulatory Pathway: Continued clinical development with confirmatory survival analyses pending.
Safety
- Overall Safety Profile: encorafenib plus cetuximab combined with FOLFIRI demonstrated a safety profile consistent with the known toxicities of the individual agents. No new safety signals were observed.
- Grade ≥3 Events:
- encorafenib + cetuximab + FOLFIRI: 39%
- FOLFIRI +/- bevacizumab: 37%
- Treatment discontinuation:
- Combination arm: ~10%
- Control arm: ~9%
- Notable toxicity patterns:
- GI toxicity (nausea, diarrhea, vomiting)
- Dermatologic effects (rash, hyperpigmentation)
- Myelosuppression
Key Clinical Implications
✔ Supports early integration of targeted therapy in BRAF V600E-mutant mCRC
✔ Reinforces the central role of targeted therapy as the primary driver of response
✔ Demonstrates chemotherapy backbone flexibility beyond oxaliplatin-based regimens
✔ Provides an option for patients in whom oxaliplatin-associated peripheral neuropathy is a concern
✔ Achieves rapid and durable responses in a high-risk population
Bottom Line
Cohort 3 of the BREAKWATER trial demonstrates that encorafenib plus cetuximab combined with FOLFIRI significantly improves response rates compared with standard FOLFIRI-based therapy in treatment-naïve BRAF V600E-mutant mCRC, without a meaningful increase in toxicity. Ongoing follow-up will determine whether these improvements translate into durable progression-free and overall survival benefits.
Sources:
- American Society of Clinical Oncology. Combining encorafenib and cetuximab with FOLFIRI may be effective first-line treatment for advanced colorectal cancer. ASCO Press Center. January 2026.
- ClinicalTrials.gov. NCT04607421: BREAKWATER – Encorafenib plus cetuximab, with or without chemotherapy, in BRAF V600E–mutant metastatic colorectal cancer. U.S. National Library of Medicine.
- Pfizer Inc. Pfizer’s BRAFTOVI® regimen with additional chemotherapy backbone increased response rates for certain patients with metastatic colorectal cancer. January 10, 2026. Press release.