Latest Updates in Genitourinary Oncology
Dr. Katie S. Murray, DO on Evolving Treatment Strategies for NMIBC
Dr. Katie Murray discusses the diagnosis and management of non–muscle invasive bladder cancer, emphasizing cystoscopy and initial TURBT as the...
ZIRCON-X: TLX250-CDx PET/CT Changes Management in Indeterminate Renal Masses
Indeterminate renal masses (IRMs) are a common diagnostic challenge, often leading to invasive biopsies, unnecessary surgery, or prolonged surveillance due...
Targeting HIF‑2α and VEGF in Post-Immunotherapy RCC: Belzutifan + Lenvatinib Clinical Insights
The combination of belzutifan (WELIREG) plus lenvatinib (LENVIMA) met the primary endpoint of progression-free survival in the Phase 3 LITESPARK-011...
Rucaparib FDA Approval: New PARP Inhibitor Options for BRCA-Mutated mCRPC
The FDA has granted full approval to rucaparib, an oral PARP inhibitor, for adults with BRCA1/2-mutated metastatic castration-resistant prostate cancer...
EMBARK Phase III: Enzalutamide Plus ADT Improves Survival in High-Risk Biochemically Recurrent Prostate Cancer
High-risk biochemically recurrent (hrBCR) prostate cancer represents a clinically challenging disease state, characterized by rising prostate-specific antigen (PSA) levels despite...
Phase 3 AMPLITUDE Trial Supports FDA Approval of Akeega in mCSPC
FDA approves Akeega (niraparib + abiraterone acetate with prednisone) for BRCA2-mutated metastatic castration-sensitive prostate cancer, showing significant improvements in radiographic...
Latest in Renal Cell Carcinoma
ZIRCON-X: TLX250-CDx PET/CT Changes Management in Indeterminate Renal Masses
Indeterminate renal masses (IRMs) are a common diagnostic challenge, often leading to invasive biopsies, unnecessary surgery, or prolonged surveillance due...
Targeting HIF‑2α and VEGF in Post-Immunotherapy RCC: Belzutifan + Lenvatinib Clinical Insights
The combination of belzutifan (WELIREG) plus lenvatinib (LENVIMA) met the primary endpoint of progression-free survival in the Phase 3 LITESPARK-011...
FDA Issues CRL for Telix’s Breakthrough Kidney Cancer Imaging Agent TLX250-CDx Over Manufacturing Concerns
The FDA has issued a Complete Response Letter for TLX250-CDx (Zircaix), rejecting the biologics license application for this breakthrough therapy-designated...
The Effect of GLP-1 Receptor Agonists on Outcomes in Metastatic Renal Cell Carcinoma Patients Undergoing Immune Checkpoint Inhibitor Therapy
GLP-1 agonists in mRCC patients showed 51% lower mortality and reduced immune toxicity with checkpoint inhibitors in 994-patient analysis.
68Ga-NY104 PET/CT Outperforms 18F-FDG PET/CT in Metastatic ccRCC
68Ga-NY104 PET/CT shows better diagnostic efficacy than 18F-FDG PET/CT in patients with metastatic clear cell renal cell carcinoma.
Latest in Bladder Cancer
IMvigor011: Signatera™ ctDNA Testing Guides Adjuvant Atezolizumab Benefit in Muscle-Invasive Bladder Cancer
Signatera-positive patients treated with atezolizumab had statistically significant improvements in disease-free and overall survival.
KEYNOTE-905: First Perioperative Therapy to Demonstrate Survival Benefit in Cisplatin-Ineligible Muscle-Invasive Bladder Cancer
First positive Phase 3 trial shows perioperative immunotherapy combination addresses critical gap for cisplatin-ineligible patients.
Sasanlimab-BCG Combination Achieves 32% Risk Reduction in BCG-Naïve High-Risk NMIBC: Phase 3 CREST Trial Results
32% risk reduction in high-risk bladder cancer with sasanlimab-BCG combo—first treatment advance in 30+ years for BCG-naïve NMIBC patients.
FDA Approves Gemcitabine Intravesical System (Inlexzo) for BCG-Unresponsive Bladder Cancer
FDA approves gemcitabine intravesical system for BCG-unresponsive bladder cancer, showing 82% complete response rate in SunRISe-1 trial.
Ideaya Advances Precision Oncology Pipeline with Positive Data from Three Cancer Drug Candidates
Ideaya presents promising data across three cancer drugs: darovasertib (76% response), IDE849 (58% response), and IDE397 combo (57% response).
TAR-200 Achieves 82% Complete Response in BCG-Refractory Bladder Cancer: SunRISe-1 Results
New drug-releasing system, TAR-200, eliminated tumors in 82% of patients in phase 2 clinical trial for high-risk bladder cancer.
Latest in Urothelial Carcinoma
Dr. Katie S. Murray, DO on Evolving Treatment Strategies for NMIBC
Dr. Katie Murray discusses the diagnosis and management of non–muscle invasive bladder cancer, emphasizing cystoscopy and initial TURBT as the...
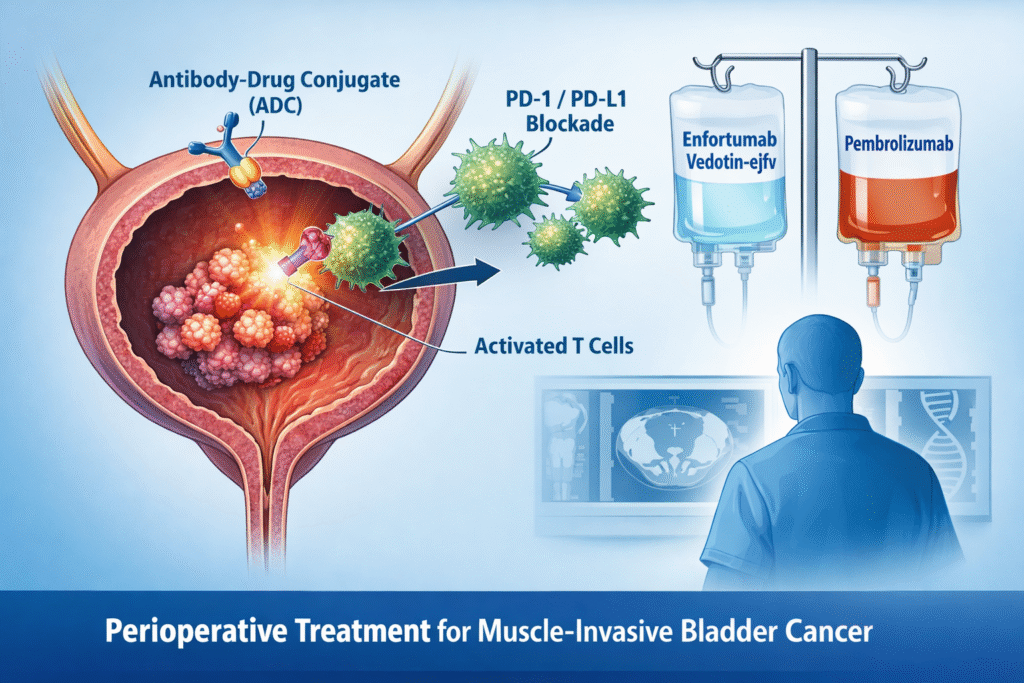
Phase III KEYNOTE-B15: Perioperative Enfortumab Vedotin-ejfv + Pembrolizumab Improves Outcomes in Cisplatin-Eligible Muscle-Invasive Bladder Cancer
Phase III KEYNOTE-B15 data demonstrate significant improvements in event-free survival (EFS), overall survival (OS) and pathologic complete response (pCR) rates...
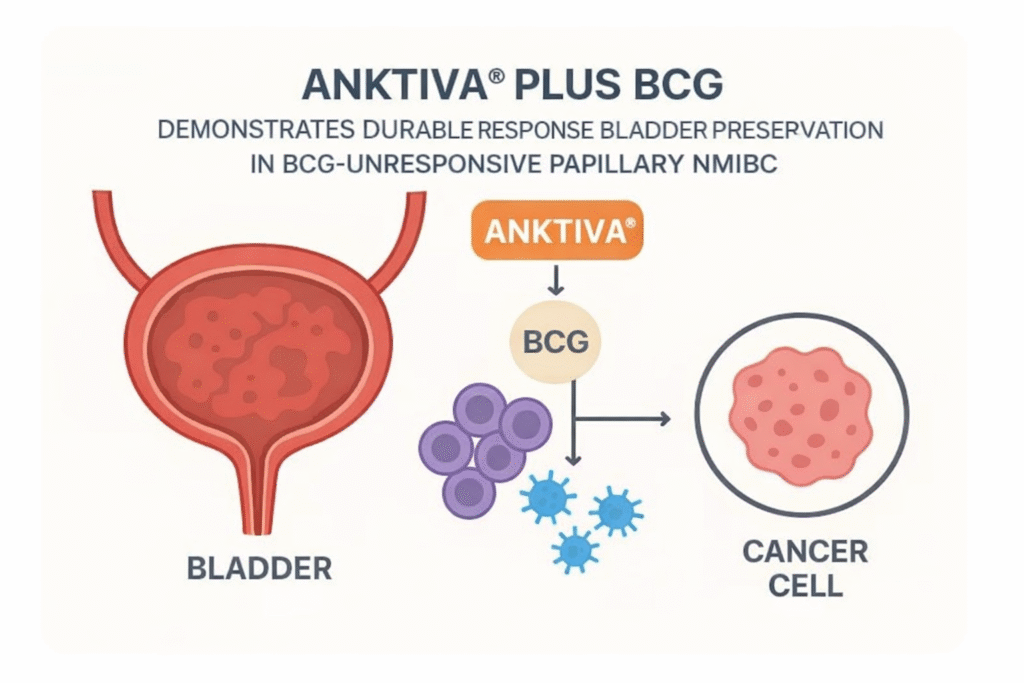
ANKTIVA® Plus BCG Demonstrates Durable Response, Bladder Preservation, and 96% Survival in BCG-Unresponsive Papillary NMIBC
This article reviews long-term outcomes of the IL-15 receptor agonist nogapendekin alfa inbakicept (NAI) combined with intravesical bacillus Calmette-Guérin (BCG)...
A New Era in MIBC: Perioperative Pembrolizumab + Enfortumab Vedotin Approved for Cisplatin-Ineligible Patients
On November 21, 2025, the U.S. Food and Drug Administration approved pembrolizumab (Keytruda or Keytruda Qlex) combined with enfortumab vedotin-ejfv...
Prospective DRIVE Study Demonstrates Strong Diagnostic Performance of CxBladder Triage Plus in Hematuria Evaluation
The CxBladder Triage Plus DRIVE study showed high accuracy in detecting urothelial carcinoma in patients with hematuria, with 94% sensitivity...
New CMS J-Code Set to Enhance Adoption of Mitomycin Therapy for Recurrent LG-IR-NMIBC
CMS has assigned a permanent J-code (J9282) to ZUSDURI, an intravesical mitomycin therapy for recurrent low-grade, intermediate-risk NMIBC, effective January...
Latest in Prostate Cancer
Rucaparib FDA Approval: New PARP Inhibitor Options for BRCA-Mutated mCRPC
The FDA has granted full approval to rucaparib, an oral PARP inhibitor, for adults with BRCA1/2-mutated metastatic castration-resistant prostate cancer...
EMBARK Phase III: Enzalutamide Plus ADT Improves Survival in High-Risk Biochemically Recurrent Prostate Cancer
High-risk biochemically recurrent (hrBCR) prostate cancer represents a clinically challenging disease state, characterized by rising prostate-specific antigen (PSA) levels despite...
Phase 3 AMPLITUDE Trial Supports FDA Approval of Akeega in mCSPC
FDA approves Akeega (niraparib + abiraterone acetate with prednisone) for BRCA2-mutated metastatic castration-sensitive prostate cancer, showing significant improvements in radiographic...
Genomic Risk Classifiers in Prostate Cancer: Precise Tools Awaiting Clinical Standardization
GRCs for prostate cancer show promise but lack standardization, with usage patterns varying by income and geography in clinical practice.
Decipher Test Identifies Prostate Cancer Patients Who Benefit Most from Chemotherapy
Molecular profiling identifies prostate cancer patients who benefit most from chemotherapy, sparing others from unnecessary side effects.
FDA Expands Pluvicto Indication as PARP Inhibitors Advance Biomarker-Driven Prostate Cancer Strategies
FDA expands Pluvicto for earlier use while PARP inhibitors show benefit in BRCA1/2+ patients, advancing precision prostate cancer care.